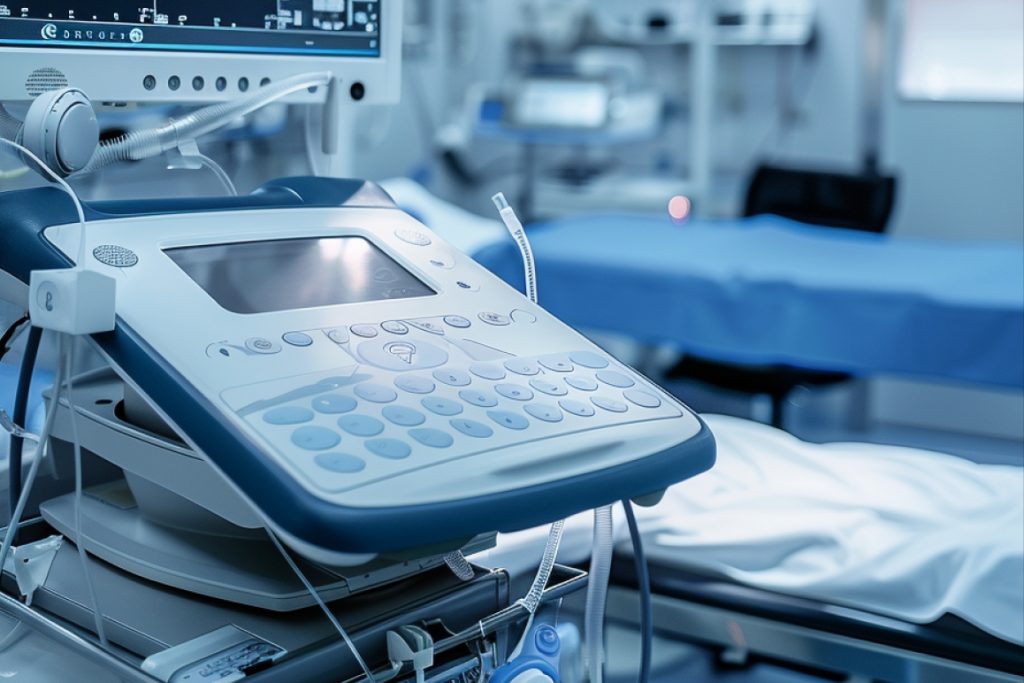
MedTech Industry
The medical device (MedTech) sector in the European Union encompasses a wide range of products and services aimed at improving patient care and quality of life. This includes diagnostic equipment, surgical instruments, implants, imaging systems, healthcare IT solutions, and in vitro diagnostics (IVDs). MedTech innovations support early diagnosis, minimally invasive procedures, and more effective therapies.
Types of Certified Medical Devices
Under EU regulations, certification covers medical devices (MD) and in vitro diagnostic devices (IVD). The process involves risk-based classification, preparation of technical documentation, and rigorous reviews and audits conducted by notified bodies.
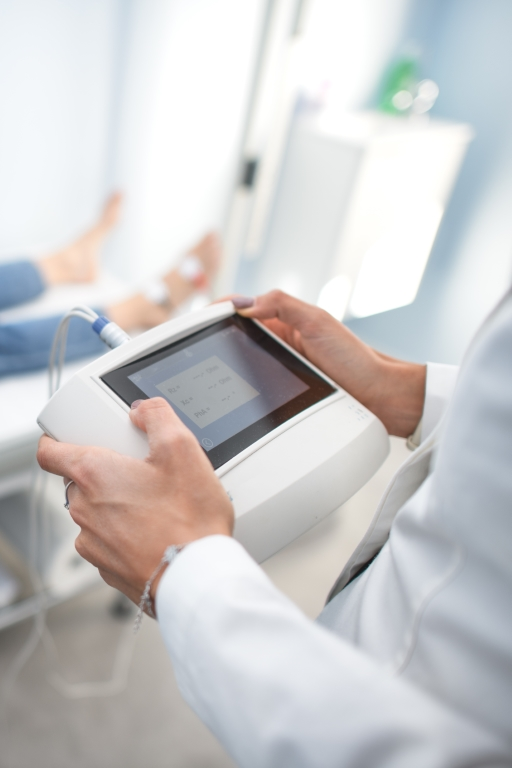
Services for the MedTech Industry
As a future EU Notified Body, we offer:
- MDR and IVDR certification
- Quality management system certification (ISO 13485)
- MDSAP audits
- Training and webinars
- Organization of industry events